On the oddities of Quantum Physics - Part 2
In my old post What is real I introduced you in the fascinating world of Quantum Physic. In this post I am going to continue the exploration of the subatomic world and a lots of new interesting things. In particular, I’ll show you that the main difference between Quantum Mechanics and Classic Mechanics is evident when we observe a subatomic particle that moves from point A to point B.
Classical mechanics, also called Newtonian Mechanics in honor of the great physicist Isaac Newton, is based on the principle that if we know the initial position, the velocity and all the forces acting on a generic object, we are able to predict its evolution in the future. We say that the particle follows a trajectory because at each time we know its position, velocity, and direction. This concept of trajectory may seem apparentely trivial and obvious, but is extremely important in the classic conception of the world.
Indeed, in 1766 Pierre Simon Laplace stated that if we knew the position and velocity of all particles in the universe, it would be possible to predict the evolution for eternity. The ignorance of the state of the system and its complexity prevent us from achieving this goal with certainty respect to the vast majority of phenomena.
By principle, know a trajectory is equivalent to predict the future!
Unfortunately, the point of view of Quantum Mechanics about the movement of an object is totally different. We can’t know exactly and simultaneously the position and the momentum (which is the velocity multiplied by the mass) of a subatomic particle.
But this is not all. Indeed, there is an uncertainty about where the particle is going and if it starts at the point A and then arrive to the point B, noone can say exactly how it went from A to B. All the paths should be considered and the result is the sum all the contributions of each path .This approach is known as Path Integral and was introduced by Richard Feynman, one of the best scientists of the last 21th Century.
The concept of Path Integral is useful to explain a lot of strange things that could happen in the quantum world and that are beyond our conception of the physical worlds. For instance, we can use this tecnique to explain how a single electron or photon can pass from both holes in a double slit experiment.

######Path Integral (Credit: http://en.wikipedia.org)
###The collapse of the classical view of the world
The first topic I would explain in this article is what is meant for Classical Physics. In the early years of the twentieth century, Physics were based on three main pillars:
-
Classical Mechanics: concerns the movement of material objects as determined by the forces acting on them, which are manifested as attractions or repulsions between mutual particles,
-
Electomagnetism: concerns the electric and magnetic phenomena based on the concept of the electromagnetic field,
-
Thermodynamics: concerned with object’s heat and temperature and their relation to energy and work.
Scientists had the opinion that everything in the Universe was composed by waves or particles, and the physical reality had three essential characteristics:
- determinism
- properties are objectively owned
- continuity (natura non facit saltus)
However, as explained in my old post, with this theoretical apparatus the physicists weren’t able to explain some fundamental phenomena:
-the dependence of the color from the temperature of the things: for istance, a bar of iron heated to produce heat change its color
-atoms and their properties: in any way an atom of a certain substance is produced, always exhibits the same characteristics that determine uniquely the behavior in the physical and chemical processes which it takes part
-stability of the atoms: according to the laws of electromagnetism, the charges which are subject to move in an elliptical trajectory must radiate, and consequently losing their energy quickly so their orbits should collapse on the nucleus.
It was in the early thirties of 1900, thanks to the insights of brilliant theoretical physicists like Planck, Einstein, de Broglie, Bohr, Heisenberg and Schrodinger that Quantum Physics was born.
Quantization is a fundamental property of electromagnetic radiation
####Planck’s hypothesis
The first brilliant idea came in mind to Plank which hypothesized that the exchange of energy E between radiation and matter does not occur continuously but in discrete multiples of the amount 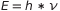 , where
, where 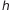 is a universal constant called Planck’s constant and its value is 6.62606957×10−34 Js.
is a universal constant called Planck’s constant and its value is 6.62606957×10−34 Js.
In 1905, Einstein had applied a similar assumption to explain the photoelectric effect, assuming that the quantization is a fundamental property of electromagnetic radiation.
####Bohr’s model of the Atom Bohr suggested the quantization of atomic energies and explained for the first time the fact that atoms emit and absorb radiation only in very specific frequencies. This means that not all the possible orbits can be traversed by electrons. Indeed, electrons can only move in special orbits without irradiating. For these orbits there are defined energy values.
####de Broglie’s hypothesis
Even the matter possesses wave properties. For any particle which have mass m and velocity v is associated a wave of wavelength 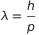 .
where h is Planck’s universal constant and p is the momentum
.
where h is Planck’s universal constant and p is the momentum 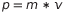 .
.
In this way, the only possible orbits for an electron moving at velocity v are those that assume a stationary configuration (i.e. are such that an integer number of wavelengths are exactly the length of an orbit).
Therefore, after the first 20 years of 20th century, in addition to the waves and the particles, it was clear that the Nature was also composed by elements that behave sometimes as waves and sometime as particles, a sort of wave-corpuscles.
The Classical Physics explained very well how particles behave (kinematics, dynamics) and waves (elasticity, electromagnetism), but didn’t not know how to handle these new wave-corpuscles. Quantum Physics was born to give an explanation of these new strange things (for know more read my old post What is real).
####Heisenberg’s Uncertainty Principle
Conceptually, the observation of a subatomic particle is not different from the observation of a normal object. Of course, the scales are really smaller, and it is for these reasons that the experiments are carried out in advanced laboratories such as CERN and LANL, using the most advanced technologies of the world.
In 1927, the German physicist Werner Karl Heisenberg enunciated his famous Principle of Uncertainty that marked, in a sense, the principal cause of the collapse of the classic vision of the world.
It is not too much complicated understand what are the foundations of this principle and to explain how it works.
Suppose you have an object and you want to measure the trajectory it is following. If you are thinking that the first thing to do is know its position and its speed, well you are right. Now, suppose also that your object is incredibly smaller like an atom, an electron or a quark.
To see this particle, you must make sure that it spreads the light that bump on it in a way that a part of the widespread light can reach your eyes or, more generally, an instrument for the revelation. To do this it is necessary that the wavelength of the light used is at most the same size of the object that you want to observe. But if you had read my old post, you know the photons that make a beam of light with short wavelength have also much energy. So, what happens is that the photon interact with the material particle (is called Compton effect):

######Heisenberg’s microscope (Credit: http://www.physicsoftheuniverse.com)
Ultimately, the particle that you are able to see because a photon has reached the detector, has suffered a shock which has accelerated it randomly. After the measurement you can know what was its position, but at the same time you have lost all possibilities to accurately determine the momentum.
It’s interesting to note that decreasing the uncertainty p using light with a greater wavelength, reduce the energy of the incident photons but consequently increases the uncertainty about the position of the particle you want observe.
The lesson you learned is that you cannot observe a subatomic particle without introducing any new element in the situation, e.g. a molecule of water vapor that can hit the particle, or a photon. These elements possess their own energy and thus produce an effect on what they hit, the thing that you want to observe! In subatomic world, the detector is playing an active role in the process of measurement.
In subatomic world, the detector is playing an active role in the process of measurement.
Starting from this point, it should be easy figure out why laws and the predictions of Quantum Mechanics are statistical. Nobody can never predict exactly the result of a single measurement of any atomic process, but only predict the probability of a result is in a range of possibilities. This means that the Law of Causality ceases to be valid!
In the rigorous formulation of the causal law - if we know the present we can calculate the future - here, it is not the conclusion wrong, but the premise.
Pointing with 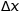 and
and 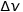 respectively the imprecision on the position and velocity of a subatomic particle, we have the fundamental relation of Heisenberg’s uncertainty:
respectively the imprecision on the position and velocity of a subatomic particle, we have the fundamental relation of Heisenberg’s uncertainty:
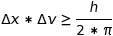
where h is Planck’s constant.
It should be emphasized that the uncertainty principle doesn’t pose any conceptual limit to the precision with which one can determine the position or the velocity. A higher accuracy on the position entails increasing imprecision on speed. Similarly, an experimental procedure whom aimed to provide a more precise knowledge of the speed, implies to lose knowledge of the position.
The uncertainty principle is a direct and inevitable consequence of the peculiar dual wave-particle nature of all physical processes. The position and velocity are incompatible physical variables, that are subject to the uncertainty principle. Note that not all of the physical variable are mutually incompatible. For instance, the positions of a particle along a direction and along another orthogonal direction are compatible.
The key concept of causality of classical physics, the of trajectory, is lost here!
In fact, only if a particle has, in each instant of time, position and speed well defined, it follows a precise trajectory. But since the uncertainty principle shows that it is impossible to measure simultaneously the two variables with the desired accuracy, the concept of trajectory does not exist!
####The Complementary Principle
In 1927, Bohr developed the Complementarity Principle, according to which in the description of the nature of the microphysical processes come into play mutually exclusive but complementary aspects, such as the wave and corpuscular aspect of light.
The inability of the investigator to take into account simultaneously these aspects during the act of the measurement is the origin of the random nature of the Quantum Mechanics’ Probabilistic Laws.
Toghether with Uncertainty Principle, the Complementary Principle is a pillar of one the ufficial interpretation of the Quantum Mechanics, the Copenhagen interpretation.
####The Schrodinger’s cat
The so called paradox of Schrodinger’s Cat is a famous thought experiment proposed in 1935 by the German physicist Erwin Schrodinger. The purpose of this experiment was to demonstrate the limits of quantum mechanics in describing a macroscopic system.
Schrodinger hypothized to put a cat and a single atom of a radioactive element into an isolated container. He added a small vial of poison connected to a radiation detector (e.g. a Geiger counter) able to measure if the decay had occurred or not. He imagined to seal the container and after a while he wondered if the cat was alive or dead.

######Schrödinger’s Cat (Credit: http://en.wikipedia.org)
Predict whether a single atom is decayed or not is undecidable. It may be take a millisecond or few billion of years. Being undecidable, at any time after the container has been closed, there is a 50 % probability that the atom has decayed and 50% that the atom is still there. So the poor cat in the container has a 50% chance of being alive and 50% to be dead. If you are forced to make a decision, you would argue that the cat is half alive and half dead.
How to determine uniquely this? We have to open the container, but now the cat is no longer in a sealed container but in a opened container. Now we can tell if the cat is alive or dead, but in this way we have perturbed the system.
The quantum principle parallel to the argument just made is called the Quantum Superposition principle.
Quantum mechanics, indeed, is perfectly deterministic in its interior, since the wave functions that describe the state of the system evolves over time according to the Schrodinger’s equation. Given the current state, Schrodinger equation allows to calculate exactly the future state of the system.
There is no uncertainty at this level, the only limit is that, in the classical vision, there are some interesting states of the system (e.g. a particle is located exactly at a certain point and has exactly a certain speed ). However, we have seen before that these status in quantum mechanics don’t exist.
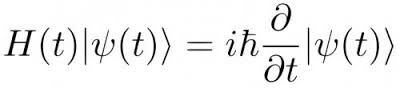
######Schrödinger’s Equation (Credit: http://en.wikipedia.org)
Quantum mechanics predicts overlapped states. Each system, such as a body in motion, can be thought of as a superposition of different states each with different energy and speed. Some states are more probable then others. These superpositions states are impossible in classical mechanics and they can be counterintuitive (e.g. there may be a particle that is “simultaneously” in two different places).
In general, each of these states is well defined and separated from the others (e.g. they own specific energy). This separation is called quantization.
The uncertainty arises up when the system need to interact with the classic world. This interaction is generally called a perturbation.
Perturb a system means that we force it to assume one and only one state. In general, a perturbation which we are interested in is that due to the measurement instrument, which is responsable of the collapsing of the wave function. To put it in simple words, the so strange status overlapping that I mentioned above, disappears.
To continue with our example, we say that the particle is found in only one of two possible places, with a certain probability, which is the one to which we refer when we say that the quantum mechanics is “probabilistic”.
This collapse, however, is very strange. If the interaction of the system with a measuring instrument must change evolution in a probabilistic manner, why the interaction with any other quantum object also changes the evolution but in a deterministic way?
What does make a measuring instrument different from another object? In the end, a measuring instrument must comply with the same physics laws of the system, and then, if Quantum Mechanics is correct, also the measuring instrument must behave accordingly the same laws.
So the collapse of the wave function shouldn’t happen, and the measuring instrument has a superposition of states. As a consequence, this means that the cat is both alive and dead in the same time!
As said before, with this paradox Schrodinger wanted to demonstrate the contradictions of quantum mechanics to describe the macroscopic reality.
####Using Probabilities in Phisics In classical physics, probability is present and can be considered due to our ignorance of the real state of the physical system. We call it *epistemic** probability becasue it is due to our ignorance.
In quantum world, however, probabilities aren’t epistemic. If we assume that the quantum description of the physical systems is valid and complete, the quantum probabilities can not be attributed to our ignorance, but to a lack of information about the system.
It should be noted that this impossibility is not practical or technological, nor authorizes us to think that the particle has these values and that is simply forbidden to us their knowledge simultaneously. We could say instead that if the particle has a precise position, then it has no well-defined speed! It is not a problem of our ignorance but of ontological indeterminism.
Anyone who is not shocked by quantum theory has not understood it (N. Bohr)
At this point we can synthesize the conceptual framework of the Quantum Theory:
- Quantization
- Indeterminism (wave-corpuscles)
- Probability non-epistemic
The quantum indeterminism changed totally the conception of the Universe: the physical events are genuinely random, or some parameters are not, in principle, knowable nor manageable.
For many physicists this was disconcerting. The quantum world was presented as an acausal world, governed by probabilistic laws where waves and particles can be turned into each other. The realism which classical physics was founded on, after nearly three centuries wavered.
Einstein thought that the quantum physic was totally unacceptable! He spent the last part of his life to prove that something was wrong in quantum physics. And in this occasion he said it’s famous sentence: God doesn’t play dice with the Universe.
###Conclusion We have seen that Quantum mechanics is a theory formulated in the 30’s (around 1927), with the help of many physicists, Planck, Bohr, Schrodinger, Heisenberg, Pauli, Jordan, Born, Dirac and others … and also thanks to the “special” contribution given by Einstein (and his acute criticism).
Today the theory is largely accepted: there are a formalism and axioms that govern it and combining it with reality. However, this formalism explains the microscopic reality but in his own way.
Indeed, the theory works perfectly: his formalism has allowed us to make very precise predictions, but absorbs within himself (elevates at principles) the idea that we should give up some categories on which it was based the explanation of the phenomena of classical physics:
- Principle of Causality (determinism)
- Representativeness of phenomena in space
- Principle of Non-contradiction (an object is one thing or another one)

We have also seen that there is a strong link between the quantum theory and measurements. The theory stipulates, in fact, in a probabilistic sense, the possible outcomes of the measures. Because of this Einstein, Schrodinger and Bell have argued that quantum theory speaks only of what we will find if we perform a measurement and not what exists out there.
The fact that the theory is orientated on the possible outcomes of the measures at the expense instead of what happens during the physical process, which remains somewhat obscure, was effectively illustrated by John Wheeler with the image of a nebulous dragon, which are seen well the tail and the head, but whose body is hidden.

######Nebulous dragon
Equally quantum physics can, for example, as we have seen, to know the initial situation of a photon detector and find out where it ends, but the journey that route remains a complete mystery.
###Further Information
More of the information in this article comes from the wonderful book The Search for Superstrings, Symmetry, and the Theory of Everything written by John Gribbin. Below more interesting lectures:
The Road to Reality: A Complete Guide to the Laws of the Universe, by Roger Penrose
Dance of the Photons: From Einstein to Quantum Teleportation
Quantum mechanics, Wikipedia.org
Photon, Wikipedia.org