On the oddities of Quantum Physics - Part 1
In this post I discuss the fascinating discoveries and the intellectual challanges that the physicists have done during the last century to bring the knowledge of the Universe over the limit until then known. Reading this article you’ll discover that subatomic world do not obey the Newton’s Laws that can be applied to apples, pears and enormous mass like planets. Electrons, protons, neutrons and all the others sub-atomic particles obey the laws of the Quantum Physics (QF), where the particles behave like waves and waves like particles, where nothing is certainly and the probability laws govern everything … this is so strange and so amazing (and gives also really big sore head)!
Our roadmap is (quite) easy… I’ll start with a brief introduction of the atom model and the main characteristics of the QF. Then we’ll see how QF is born starting from the classical concepts of matter and light. We’ll discuss some famous experiments and, finally, explain the implications of the theory in our lifes.
Like a Lego, everithing is mades by tiny (but really cool) parts
Quantum physics appeared in the early years of twentieth century as a result of the studies of the most important physicists of the time as Max Planck, Albert Einstein and Erwin Schrödinger. It was more than a new theory to describe the sub-atomic world. It really caused a conceptual earthquake that posed serious problems about the conception of the world and the reality.
Indeed, at the end of 1800 the idea that everything in the Universe is made up of tiny particles was not a novelty. This idea goes back to Democritus, an ancient Greek philosopher lived around 400 BC. Democritus said that everything in the world is composed of atoms which are physically, but not geometrically, indivisible. They have always been, and always will be, in motion. There is an infinite number of atoms and kinds of atoms, which differ in shape and size. However, only during the nineteenth century that chemists were able to prove that Democritus’ Theory was effectively right.
The model of the atom as it is known today dates back to the studies of Ernest Rutherford in 1911 and James Chadwick in 1932. An atom can be thought like a miniature solar system with electrons orbiting around a massive nucleus. The positive charge is concentred inside a heavy nucleus, surrounded by a cloud of electrons which carry the negative charge. The dimensions we are talking about are incredibly small: a nucleus is ~10-13 cm while an atom ~10-8 cm.

######The Rutherford atomic model (Credit: Encyclopædia Britannica, Inc.)
Ernest Ruthefort proved also how the atoms of an element can turn into atoms of another element. During this process, called radioactivity, the atoms can emit two types of radiations, respectively called alpha and beta radiations. The atom’s chemical properties determine the large amount of elements found in nature. These properties depend in turn by the cloud of electrons around the nucleus, which is in such way the visible face that every atom shows to the other ones. Indeed, atoms with identical chemical properties have the same number of electrons and protons, but could have different masses due the different number of neutrons. These kind of atoms are called isotopes.
We were almost there…
During the last years of nineteenth century seemed that theoretical physics had served its purpose and had arrived at destination. There was an excellent theory on the light propagation, the Newton’s Gravity Laws and, finally, the idea that everything in the Universe was made by atoms. But during the first 30 years of the 1900, all these theories have been surpassed.
Firstly, was discovered that the behavior of the light can be explained sometimes in terms of massless particles called photons, and not always in terms of waves as believed until then. Secondly, Albert Einstein showed the real nature of the Space, Time and Gravity with his Theory of Relativity. A new world vision had gone up, totally different from the oldest. This vision said that there are no pure particles or pure waves objects, but at the fundamental level everithing can be explained in terms of waves and particles (wavicles). The first upsetting result was that is impossible predict with an absolute precision the result of an atomic experiment or any other event in the Universe, and that our world is governed by chance. Another unbelievable consequence of QF is that we cannot, by principle, know simultaneously both the position of an object and its momentum (ie where it is going). These things upset the physicists of that epoch which considered the Quantum Phisics as an heresy.
A blast in the past
The best way to approach the basic of Quantum Theory is starting from Maxwell’s work on the unification of Electric and Magnetic forces. Maxwell, as like the most of other scientists which lived in the first half of 1800, was fascinated by the fact that the current flow in a wire produce a magnetic field, as well the variation of a magnet around a metallic ring produces an electric current which flow inside it. Moving electricity make magnetism and, in the same time, moving magnetism make electricity. Maxwell had the great insight to think Electric and Magnetic forces, which until then were believed separated, as two side of the same coin, an Electromagnetic force.
Maxwell wrote four equations (to tell the truth not too easy to look) which explain this relationship in a concise way:
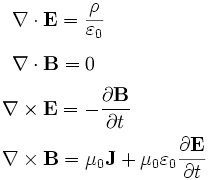
The equations have been a real success (among physicists I mean :-P ). They predicted the speed of the electromagnetic waves (about 300000 km/sec) which is the same of the light and, consequently, that the visible light is an electromagnetic wave.
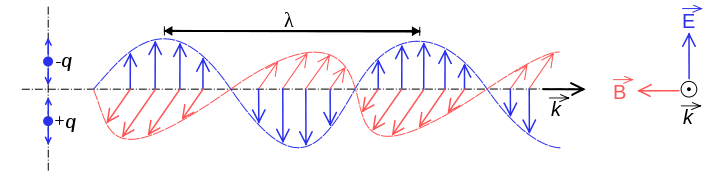
######Electromagnetic wave (Credit: http://en.wikipedia.org)
To figure out why the visible light is an electromagnetic wave, is possible observe what happen when the light interacts with itself. This crucial experiment, which removed the old idea that light is made up of particles (or corpuscles) which dates back to Newton, was performed by Thomas Young in 1800. The experiment is very simple and consists in a beam of monochromatic light which is projected through two slits placed on a wall, in order to produce two sets of waves with ridges and valleys. The interference pattern observed is very similar to the pattern resulting from the interference of water waves. In particular, in such points the contributions of both the waves are added while in other points the are subtracted. As a result there is a set of light stripes dark on the wall. One century later, however, was proved that the visible light and all forms of electromagnetic waves can also be described in terms of particles, the photons. Anyway, Newton’s initial idea was right.

######Double-slit experiment (Credit: http://en.wikipedia.org)
The first clue of the particle nature of light is due to Max Planck which first of everyone introduced the idea of discrete packets of energy. Plank was frustrated because the Physics Laws known until then weren’t unable to explain how light or any kind of electromagnetic radiations can be emitted by a body warm (also called black-body). However, after a long study, he discovered that was possible to solve this dilemma assuming that the energy had been exchanged in a discrete way. At that time it sounded like an heresy, and Plank was conscious about that. He used that mathematical trick as a pure act of desperation!
As a result he obtained a useful way to predict the nature of the black-body spectrum that could be explained assuming the light can emitted from the vibrations of the charges in the atoms only in discrete packets of energy. Moreover, this implied that the atoms could absorb energy only in small packages.
Plank proved all these relations in terms of Energy (E): so the nature of the black-body spectrum can be described as 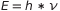 , where
, where 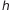 is the Planck’s constant, a very little value 6.62606957×10−34 Js. Is important underline that Max Plank does not suggest that the energy of light exists only in small packets with energy
is the Planck’s constant, a very little value 6.62606957×10−34 Js. Is important underline that Max Plank does not suggest that the energy of light exists only in small packets with energy 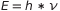 , he only said that the absorption or emission of energy can happen in a discrete way and this is something that had to do with the nature of the charges that oscillate within the object.
, he only said that the absorption or emission of energy can happen in a discrete way and this is something that had to do with the nature of the charges that oscillate within the object.
Planck’s proposal was considered no more than a mathematical trick. Nevertheless, this trick was taken seriously by the German physicist Albert Einstein, who gave him a respectable physical reality when he showed that another mystery of time could be explained thanks to it: the Photoelectric Effect.
Photoelectric effect is obtained when the light hits a metal surface in the vacuum. In this conditions the light is able to pull out the electrons from the atoms which lies in the surface. The number and the energy that these electrons carry can be detected and measured.
At that time it was not surprising that the light can pull out electrons away from the atoms. The physicists were surprised by another fact: it was clear that exists a strict relationship beetween the energy of light and the energy of the electrons pulled away. This energy depends on the light’s frequency that hits them. Einstein suggested that a light beam of frequency 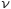 can be thinked as composed of a stream of particles, called photons, each of them with energy
can be thinked as composed of a stream of particles, called photons, each of them with energy 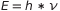 . An electron is removed from the metal when it is bumped by a photon in the good way. Each electron carries with him the total amount of energy carried by the photon
. An electron is removed from the metal when it is bumped by a photon in the good way. Each electron carries with him the total amount of energy carried by the photon 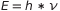 less the amount of energy needed to pull it out. Therefore, the only way to increase the energy of the electrons is increase the energy of the photon that strikes it and, consequently, its frequency.
less the amount of energy needed to pull it out. Therefore, the only way to increase the energy of the electrons is increase the energy of the photon that strikes it and, consequently, its frequency.
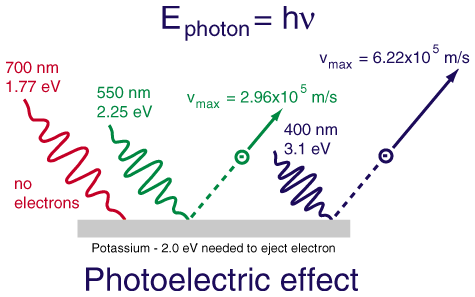
######Photoelectric Effect (Credit: http://hyperphysics.phy-astr.gsu.edu)
How I explained before, the atom model was developed by Rutheforth, whom argued that the positive charge of an atom was contained in the center (nucleus), and around it there was a cloud of electrons wich move following specific paths. Electrons move in very precise orbits and can only pass from one orbit to another one, but cannot stay in the middle. If an electron jumps from one orbit to high-energy orbit to a lower energy, a photon is emitted with energy
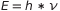 . Otherwise a photon is absorbed. Given that no longer exists something like a half photon, an electron cannot stay in the middle.
. Otherwise a photon is absorbed. Given that no longer exists something like a half photon, an electron cannot stay in the middle.
In 1924, Louis de Broglie, a French Nobleman and physicist, while he was working on PHD, had a stroke of genius: if the light waves can behave like particles, therefore also the electrons and every other sub-atomic particles can behave like a waves. The idea was initially met with skepticism, but later turned out to be right.
To prove his theory De Broglie started from both Einstein and Planck’s works and the classical physic definition of momentum. Everyone knows Einstein’s energy equation for a particle 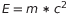 . Also Planck developed an energy equation for the photons
. Also Planck developed an energy equation for the photons 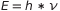 .
For a particle, is possible to define the momentum p as the product of the mass and the velocity
.
For a particle, is possible to define the momentum p as the product of the mass and the velocity 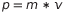 (this means that more an object is heavier and faster, more momentum it carries).
(this means that more an object is heavier and faster, more momentum it carries).
Now let’s go back to the photons. I said before that a photon moves at the same velocity of the light, wich implies it is massless. But, from the momentum definition, if a photon has no mass therefore it doesn’t carry a momentum. So there is a question to answer: if a photon does not have a momentum, how is possible that it can hit and pull out an electron from an atom?
The speed of the photon is the same of the light c, therefore Eintsten equations can be rewritten in therm of the momentum
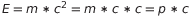 .
.
The energy is always the same, so this is equal to Plank’s energy equation. Now we have
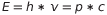 .
.
Reordering the terms we have 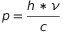 . But
. But  is the wavelength
is the wavelength 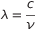 , so all can be rewritten as
, so all can be rewritten as 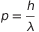 .
This is the result De Broglie was searching: for a photon, or in general every sub-atomic particle, the a momentum equals to the Plank’s costant
.
This is the result De Broglie was searching: for a photon, or in general every sub-atomic particle, the a momentum equals to the Plank’s costant 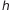 divided by the wavelength
divided by the wavelength 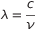 .
.
All this is extremely important. It make a link between the photon, which is a particle, with its wavelength. Coming back to the equations, terms can be reordered as 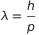 . In other words, even an electron can be viewed as a wave and its wavelength depends on the Planck’s costant and momentum.
. In other words, even an electron can be viewed as a wave and its wavelength depends on the Planck’s costant and momentum.
De Broglie was awarded the Nobel Prize in 1929, and from then on it was clear that the waves can be treated as particles and particles can be treated as waves. But do not worry too much my dear readers. This confusion, the wave-particle duality, is evident only at the atomic level and is not noticeable in our lifes. For the objects to which we are accustomed, mass involved (and therefore the momentum) are so large that it allows us to totally ignore their wave nature (the ratio 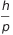 is effectively 0).
is effectively 0).
In the same years in which Einstein and Plank were working on their energy equations, the Austrian physicist Erwin Schrödinger was working on a wave equation for the electron(conceptual very similar to the Maxwell’s studies about the light), which later turned out crucial to unravel the atomic structure and explain the chemistry composition of the elements.
Schrödinger introduced the ideas of wave function 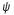 to describe the quantum state of a particle and its behaviour. Theorically, a wave function is spread along the whole Universe. The famous Shrondinger’s equation describes these wave functions and how they interact among them and, consequently, how the overall quantum state of some physical system changes with time:
to describe the quantum state of a particle and its behaviour. Theorically, a wave function is spread along the whole Universe. The famous Shrondinger’s equation describes these wave functions and how they interact among them and, consequently, how the overall quantum state of some physical system changes with time:
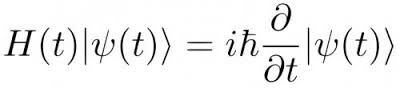
A wave function is very strong in a small region, which corresponds to a position of the subatomic particle. It becomes weaker as one moves away from this region, but continues to exist even very far from it. Shrodinger’s equation is very good to describe how electrons interfere with each other or how they interact with themselves. When we look at an electron or when try to measure them with a detector, the wave function collapses and in that precise moment we know the position of the electron. But when we stop to look at it, the wave function spreads again and interfere with other wave functions or with himself. All of this things are measurable, quantifiable and mathematically definiable: how is possible that the electrons are in atoms, how the atoms combine to create molecules and how atoms interact with themselves.

######Wavefunction collapse (Credit:http://blogs.scientificamerican.com, Image by A. Friedman)
The term collapse of the wave function has a rigorous mathematica definition; however you can think of it as a way to explain that we can know where things are only when we look at them. One look and they are gone. Fled away. Disappeared. Their behavior depends on whether or not we are looking at them.
The great lesson that comes from Shrodinger’s work, and that I’ll go deepen in the Part 2 of this post, is that in a quantum experiment the observer is part of the experiment itself, and what he chooses to watch plays a crucial role in what will happen. The implications of this are truly remarkable and profound. On one hand we can no longer say that an electron in principle be identified as a single object, which starts from a point in our experiment and follows a unique path through all of our experiment. The classic concept of trajectory, a consequence of the Newtonian Theory, is abandoned in Quantum Physics. Quantum Theory speaks in terms of events that can happen in a certain order but that does not tell us anything about what can happen to a particle in a certain event when this is not observed. All we can say is that we have observed an electron at point A and observed another electron at point B. We cannot say anything about what happened in the middle, let alone if we observed the same electron or not because electrons are indistinguishable from each other.
What is more real?
So, what is more real? The particle or the wave? There isn’t a unique answer. All depends by the question we are doing. And no matter how skilled is the physicist, there is never an absolute certainty about the answer he might have.
In the second part of this post, we will see in detail what all this means, starting from the famous Schrodinger’s cat. So try not to miss it. Finally, if you liked this post, please leaves a comment or share it.
##Further Information More of the information in this article comes from the wonderful book The Search for Superstrings, Symmetry, and the Theory of Everything written by John Gribbin. Below more interesting lectures:
The Road to Reality: A Complete Guide to the Laws of the Universe, by Roger Penrose
Dance of the Photons: From Einstein to Quantum Teleportation
Quantum mechanics, Wikipedia.org
Photon, Wikipedia.org